Abstract
Introduction: B-ALL with t(4;11)(q21;q23) (t(4;11) B-ALL) is a well characterized adult B-ALL subtype associated with an unfavorable prognosis, mainly due to a high relapse risk, which is followed by a poor outcome. In order to prevent relapse during frontline treatment, alloHCT in CR1 is recommended by many cooperative groups, although the specific outcome and prognostic factors related to this procedure have been scarcely analyzed.
Patients and Methods: We searched in the ALWP-EBMT database all adult patients (pts) (i.e., ≥18 year-old) with B-ALL reported to harbor a t(4;11) translocation who underwent an alloHCT in CR1 from a matched related or unrelated (10/10 or 9/10) donor during the 2000-2016 period, and analyzed their outcome. The prognostic value of the MRD status at the time of transplant, as reported by centers, was specifically addressed. In addition, a comparative analysis with analogous pts with normal karyotype B-ALL (NK B-ALL) was performed, adjusting for main significant variables including the pre-transplant MRD status.
Results: Overall, we identified 151 pts with t(4;11) B-ALL and 567 with NK B-ALL. Compared to NK B-ALL, pts with t(4;11) B-ALL were older (median age, 38.1 vs. 33.8, p=0.028), presented with a higher WBC count (114 vs. 7.9x109/L¸ p<.001), and were allografted within a shorter period from diagnosis (5.3 vs. 5.8 months, p=0.001). In the t(4;11) B-ALL cohort, 103 transplants were performed from an unrelated donor (68%), conditioning was myeloablative (MAC) in most cases (86%), and in 114 of them (88%) included TBI. Eighty pts (53%) received in vivo T-cell depletion (TCD) (Table 1). With a median follow-up of 62 months, in pts with t(4;11) B-ALL, the 2-year relapse incidence (RI), NRM, LFS, OS, and graft- and relapse-free survival (GRFS) were 29.8% (22-38), 19.5% (14-27), 50.7% (42-59), 59.8% (52-68), and 34.6% (27-43), respectively. Acute grade II-IV GvHD and 2-year chronic GvHD were 34.7% (27-42) and 38.5% (30-47), respectively. Outcome after relapse was poor, with a 2-year OS of only 14.9% (4-26).
MRD at the time of alloHCT was undetectable in 62 (69%) of the 90 pts with available information, and showed a strong favorable impact on outcome, with a lower RI (21 vs. 45% at 2 years, p=0.003) and higher LFS (67 vs. 27%, p=0.00004), OS (81 vs. 26%, p=0.000005)and GRFS (47 vs. 24%, p=0.01) compared to pts with detectable MRD at transplant. The prognostic value of the pre-transplant MRD status was confirmed in a multivariate analysis (performed among patients with known MRD status), in terms of RI (HR=0.23, CI: 0.09-0.55; p=0.0011), NRM (HR=0.16, 0.05-0.52; p=0.0023), LFS (HR=0.20, 0.10-0.40; p=10-5), OS (HR=0.14, 0.07-0.30; p<10-5), and GRFS (HR=0.38,0.2-0.69; p=0.001). Patient age and year of transplant also showed independent prognostic value for NRM, LFS, OS, and GRFS.
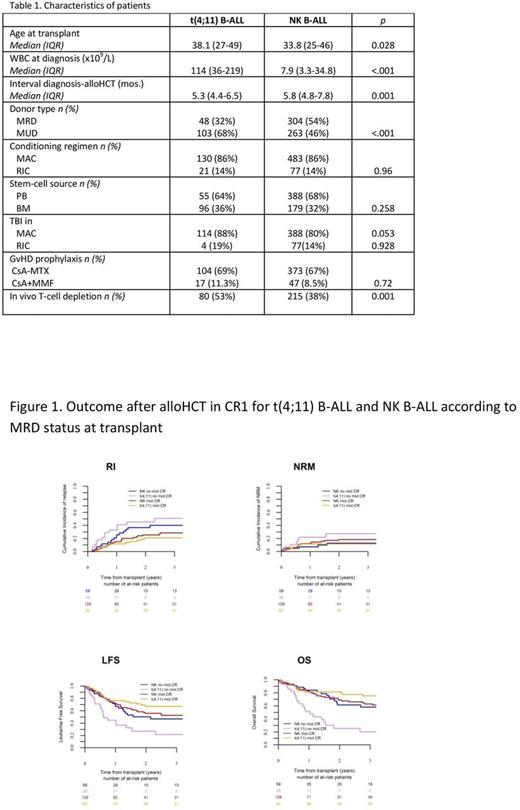
Comparison between t(4;11) B-ALL and NK B-ALL showed a strong impact of the MRD status. In univariate analysis, outcome after alloHCT in both t(4;11) B-ALL and NK B-ALL groups was similar in pts without detectable MRD at transplant, with comparable RI (21 vs. 26%, p=0.35), NRM (12 vs. 18.5%, p=0.37), LFS (67 vs. 56%, p=0.14), and OS (81.4 vs. 69%, p=0.069). In contrast, among pts with detectable MRD at alloHCT, LFS and OS were superior in NK B-ALL compared to t(4;11) B-ALL pts (50 vs. 27%, p=0.02; and 62 vs.26%, p=0.01, respectively). In multivariate analysis, and considering MRD-negative NK B-ALL as the reference category, t(4;11) B-ALL pts allografted without detectable MRD showed a better LFS (HR=0.624,0.335-0.978; p=0.04) and OS (HR=0.523,0.296-0.925; p=0.02). On the contrary, MRD-positive t(4;11) pts showed a worse outcome compared to the remaining MRD-stratified subgroups, with higher RI (HR=3.28,1.63-6.60; p=.0009), and shorter LFS (HR=2.42,1.65-4.93; p=0.00017) and OS (HR=3.51,2-6.18; p=1x10-5) (Figure 1).
Conclusions: Outcome of adult pts with t(4;11) B-ALL who received an alloHCT in CR1 is favorable, especially in those with a negative MRD status pre-alloHCT. These results, when compared to previously reported results in non-allografted pts, strongly suggest a neat beneficial effect of alloHCT for this adult B-ALL subset. Nonetheless, further studies should evaluate the magnitude of this benefit in comparison with contemporary non-allograft post-CR strategies, as well as the optimal management for pts failing to achieve a MRD-negative status.
Socié: Alexion Pharmaceuticals, Inc.: Consultancy. Schmid: Celgene: Research Funding, Speakers Bureau; MoilMed: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding, Speakers Bureau; Jazz: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding, Speakers Bureau. Mohty: Sanofi: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal